Spatial Control of Membrane Traffic
Spatial regulation of membrane traffic by microtubule-associated septins
The microtubule cytoskeleton consists of subsets of microtubules with distinct composition (e.g.,
microtubule-associated proteins, post-translational modifications) and properties
(e.g., stable, dynamic, bundled), which provide a framework that orients and helps guide membrane traffic.
This is a newly emerging principle of cell biology, and a key challenge is to elucidate the
microtubule-associated molecules and mechanisms that determine the trafficking routes of membrane
organelles/vesicles,
and determine how the topology of various microtubule subsets is established.
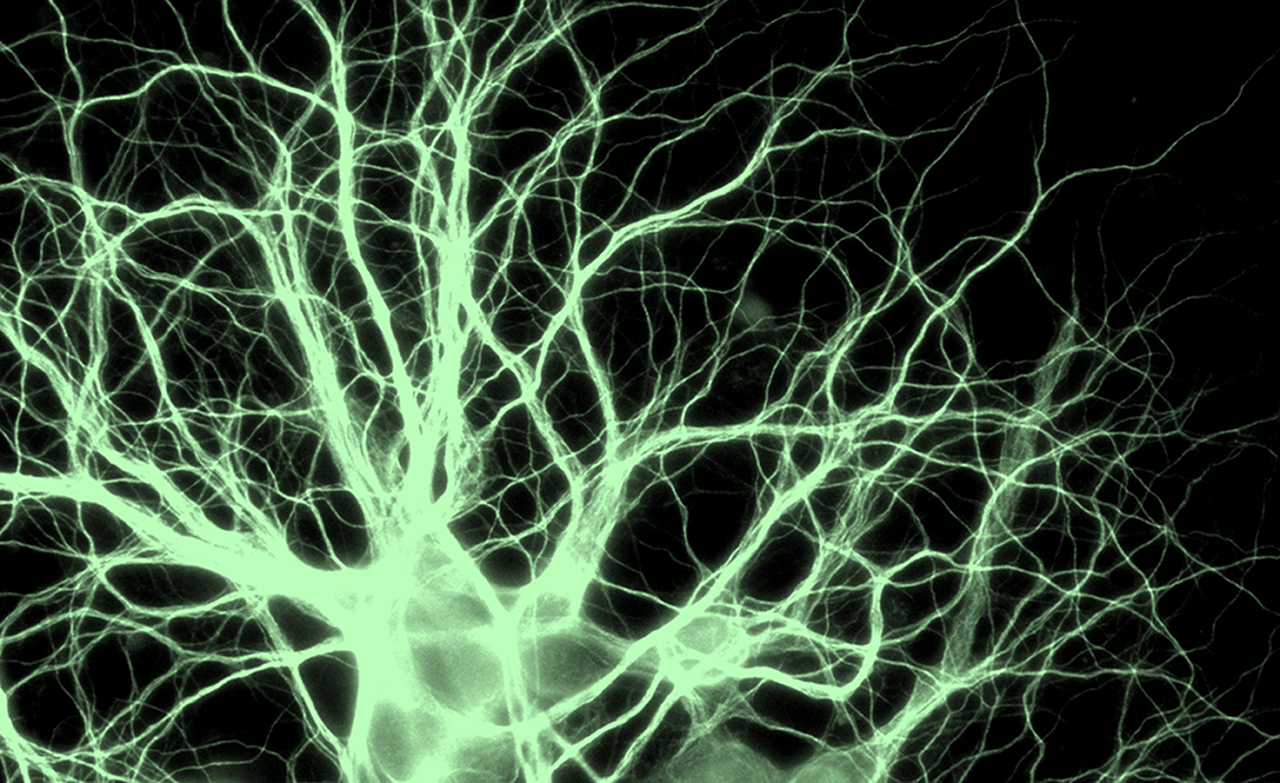
We have discovered that septins associate with distinct subsets of microtubules and are required for
microtubule-dependent transport in epithelia and neurons
(Spiliotis et al, J Cell Biol 180:295, 2008;
Bai et al, J Cell Biol 194:187).
Importantly, recent work from our laboratory suggests that microtubule-associated septins define
trafficking routes of distinct directionality and select for the transport of specific motor-cargo
Karasmanis et al, Dev Cell 46:518, 2018.
We posit that septins comprise a spatial code that directs traffic on the microtubule network, specifying the
directionality of intracellular transport in polarized cell types and processes.
On-going studies aim at mapping the localization and function of different septin paralogs and complexes in
polarized routes of membrane traffic. Using cell biological approaches and in vitro reconstitution assays
(e.g, single molecule motility assays), we investigate how septins function in microtubule orientation and
positioning, and how they regulate the movement of kinesin and dynein motors and their cargo on microtubules.
Regulation and coordination of cargo-motor interactions by membrane-associated septins
Navigation of intracellular transport involves not only MT-associated cues, but also the selective recruitment and activation/inhibition of motors by membrane vesicle/organelles.
As a single cargo is bound to multiple motors, a key challenge is understanding how membrane cargo is driven by a specific motor at a given time, and how switching from one motor to another occurs.
This is particularly critical for endolysosomal organelles, which move bi-directionally and must undergo directional targeted movements in response to metabolic and degradative signals including cell stress and injury.
The discovery of peripheral membrane proteins, which mediate motor-cargo attachments by functioning as adaptors and/or scaffolds has laid the premise for understanding how motors are recruited, activated and coordinated on organelle membranes.
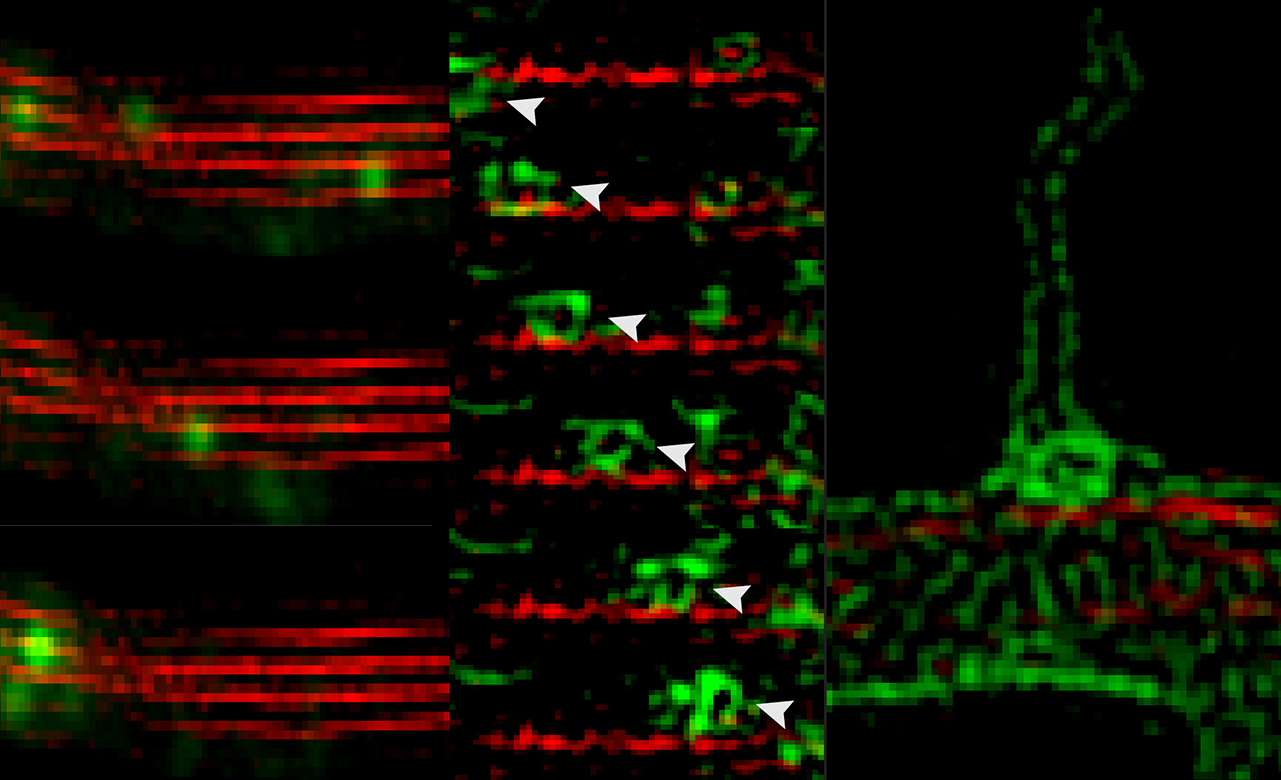
We and others have found that septins associate with endolysosomal membranes and are involved in endocytic membrane traffic, but their precise functions are little understood.
In recent studies, we found that membrane-associated septin 9 recruits dynein-dynactin and triggers retrograde traffic of endolysosomes
(Kesisova et al, bioRxiv 128488).
Interestingly, septin 9 also associates with the cargo-binding domain of the kinesin-2 motor KIF17, regulating KIF17 binding to membrane cargo proteins
(Bai et al, Mol Biol Cell 27:897, 2016).
We posit that organelle-bound septins function as scaffold and adaptor proteins for the recruitment and coordination of microtubule motors in response to signaling and metabolic cues.
On-going projects aim at determining the organelle-motor specificity and function of different septin complexes.
In addition, we are interested in deciphering the regulatory mechanisms and signaling pathways that that control septin association with membrane organelles versus microtubules - how septins may shift between cytoskeletal polymers and organelle membranes.
Neuronal Morphogenesis
Neuronal morphogenesis begins with the formation and elongation of membrane protrusions termed neurites,
which mature into dendrites and axons. Understanding the molecular mechanisms of neurite formation, growth and
differentiation is critical for the treatment of neurodevelopmental and mental health disorders such as autism
and schizophrenia, which are characterized by aberrations in neurite outgrowth.
The formation and growth of neurites is largely driven by the actin and microtubule cytoskeleton.
Spatiotemporal coordination of actin filaments with microtubules is key for every stage of neurite formation
and outgrowth. The actin cytoskeleton provides protrusive and contractive forces that crosstalk with
microtubule dynamics, which in turn feedback to and regulate actin dynamics. Capture and guidance of
microtubules along actin filaments, and conversely microtubule-based regulation of actin assembly are critical
for neurite growth and branching, and underlie the steering of neuronal growth cones in response to attractive
and repulsive cues. However, it is poorly understood how actin and microtubule dynamics are spatially
regulated and coordinated.
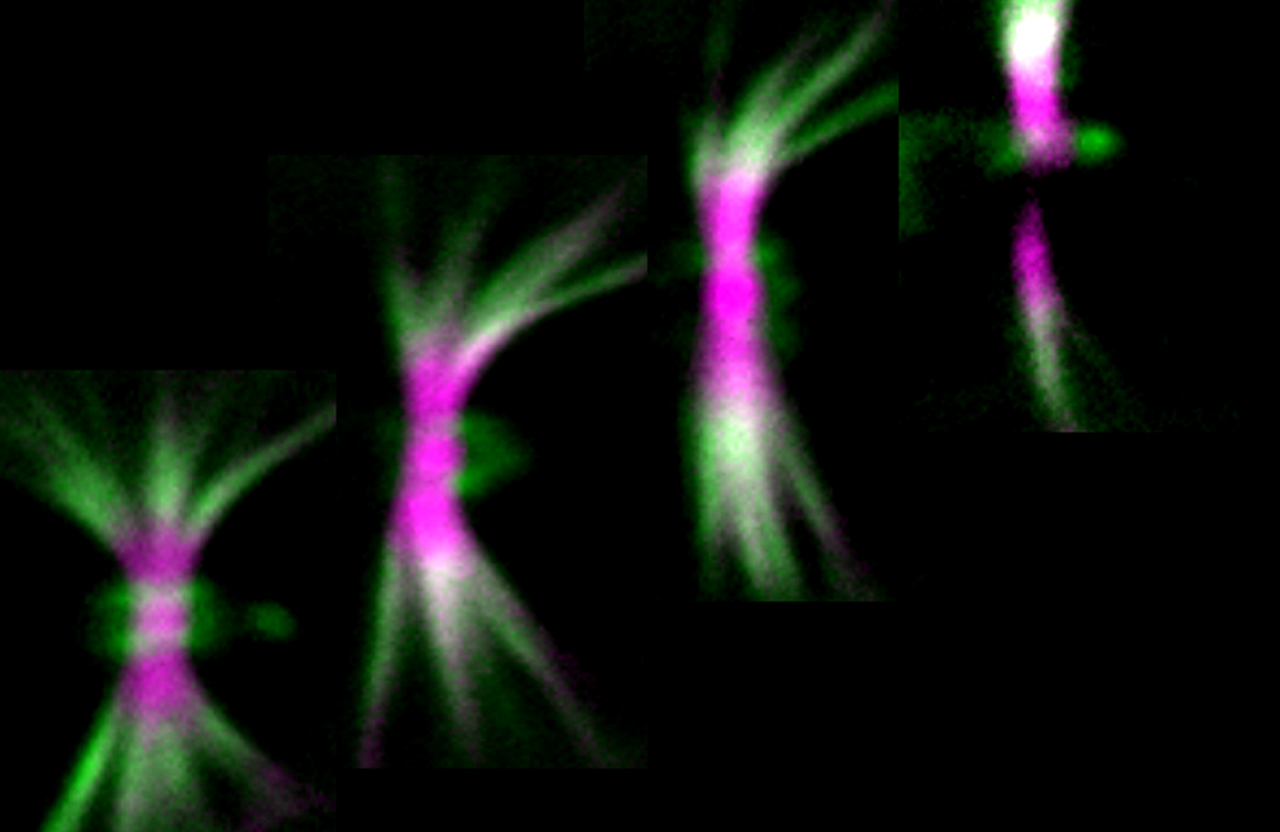
Early work from our lab showed that septins coordinate actin and microtubules during the formation of
collateral axon branches (Hu et al, Curr Biol 22:1109, 2012)
and recently, in vitro reconstitution approaches have revealed that septins link directly actin filaments with
microtubules. On-going projects focus on septin functions in the regulation of actin-microtubule dynamics and
interactions during neurite formation, elongation, differentiation and growth cone motility.
Cell Migration & Invasion
Directional cell migration underlies organogenesis and the metastasis of solid tumors. We are interested
in the molecular mechanisms that spatially control the actin cytoskeleton and its contractile and protrusive
properties in migrating epithelial cells that undergo epithelia-to-mesenchymal transition.
Septins are over-expressed and mutated in many carcinomas (Angelis and Spiliotis,
Front Cell Dev Biol
2016; Dolat et al, Biol Chem
2014).
We have found that SEPT9 is upregulated during epithelial-to-mesenchymal transition (EMT) and
SEPT9 over-expression enhances cell migration and invasion in 3D organoid and transwell migration assays
Dolat et al, J Cell Biol 2014).
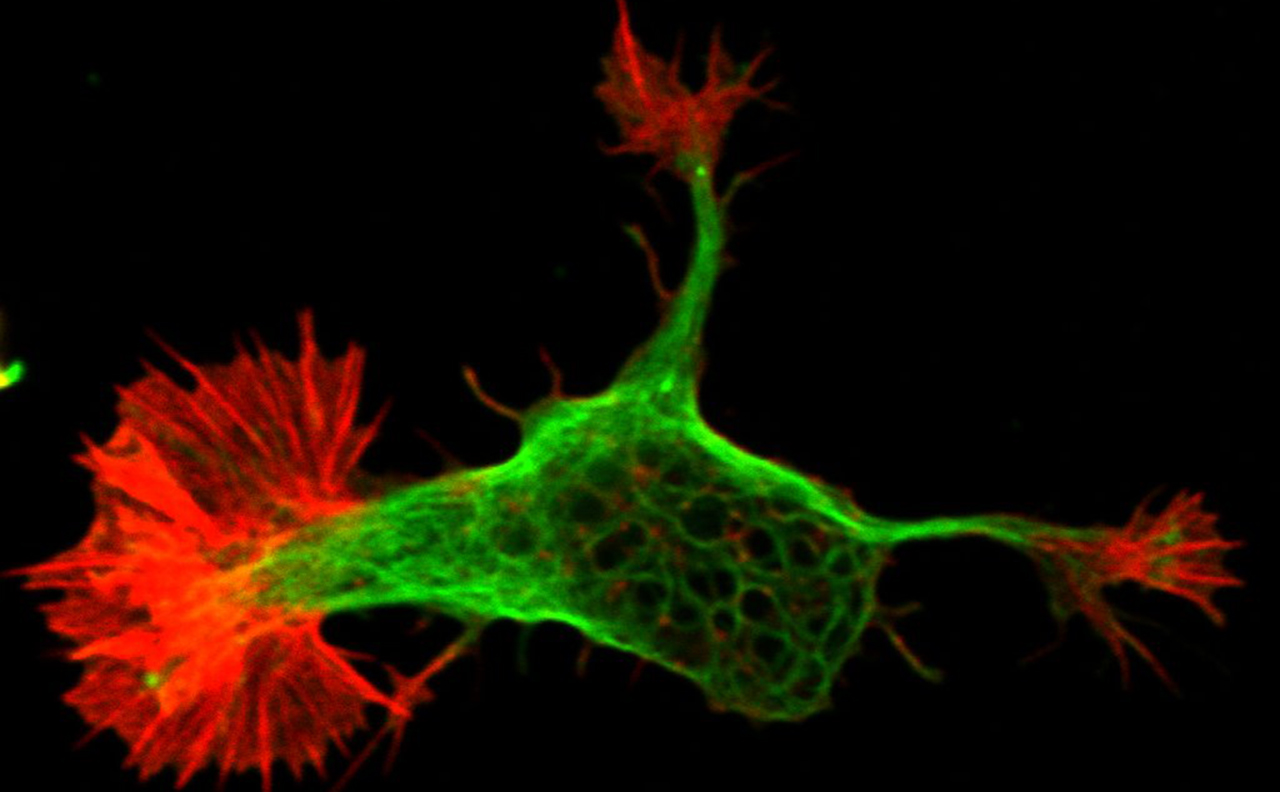
We discovered that septins are enriched at the front lamellae of migrating cells in the interface of the
transverse arc and radial (dorsal) stress fibers.
Septins are essential for the organization and maintenance of the contractile transverse arc network,
crosslinking actin filaments at the leading front of migrating cells.
This function is critical for the front-to-rear maturation of focal adhesions, which underlies directional
cell migration.
In collaboration with Dr. Shae Padrick (Drexel College of Medicine), we investigate the mechanism of
septin-actin interaction and how septins regulate actin organization and dynamics in migrating cells. In
addition, we study the role of septins in the formation and function of invadopodia, which are protrusive
structures that invasive cancer cells generate to degrade the extracellular matrix and metastasize to other
tissues and organs.